This video discusses significant figures.
Resources taken from LibreText's Introduction to Chemistry, based on textbook by Nivaldo Tro.
These chapters cover topics like Classification of matter, scientific method history of chemistry ,chemical and physical properties
Chapter 4.2: The Chemical Equation
Chapter 4.5: Composition, Decomposition, and Combustion Reactions
Lumen Learning: Chemistry for Non-Majors: 
History of Alchemy: From Lumen's Chemistry for Non-Majors
Historical Events in History of Chemistry: from Lumen's Chemistry for Non-Majors
Optional UC Davis Into to Chemistry by Nivaldo Tro
Chapter 1: What Is Chemistry?
Learn the basic terms used to describe matter. The definition of chemistry—the study of the interactions of matter with other matter and with energy—uses some terms that should also be defined. We start the study of chemistry by defining some basic terms.
Anything that has mass and takes up space. is anything that has mass and takes up space. A book is matter, a computer is matter, food is matter, and dirt in the ground is matter. Sometimes matter may be difficult to identify. For example, air is matter, but because it is so thin compared to other matter (e.g., a book, a computer, food, and dirt), we sometimes forget that air has mass and takes up space. Things that are not matter include thoughts, ideas, emotions, and hopes.
Example
Which of the following is matter and not matter?
Solution
Exercise
Which of the following is matter and not matter?
Answer
To understand matter and how it changes, we need to be able to describe matter. There are two basic ways to describe matter: physical properties and chemical properties.
A characteristic that describes matter as it exists. are characteristics that describe matter as it exists. Some of many physical characteristics of matter are shape, color, size, and temperature. An important physical property is the phase (or state) of matter. The three fundamental phases of matter are solid, liquid, and gas (Figure

Figure
: The Phases of Matter. Chemistry recognizes three fundamental phases of matter: solid (left), liquid (middle), and gas (right). Image used with permission (CC BY-SA 3.0; Spirit469)
A characteristic that describes how matter changes form in the presence of other matter. are characteristics of matter that describe how matter changes form in the presence of other matter. Does a sample of matter burn? Burning is a chemical property. Does it behave violently when put in water? This reaction is a chemical property as well (Figure

Figure
: Chemical Properties. The fact that this match burns is a chemical property of the match. Image used with permission (Sebastian Ritter (Rise0011))
If matter always stayed the same, chemistry would be rather boring. Fortunately, a major part of chemistry involves change. A physical change. A change that occurs when a sample of matter changes one or more of its physical properties. occurs when a sample of matter changes one or more of its physical properties. For example, a solid may melt (Figure

Figure
: Physical Changes: The solid ice melts into liquid water—a physical change. A time-lapse animation of ice cubes melting in a glass (50 minutes). Image used with permission (Public Domain; Moussa).
The process of demonstrating a chemical property. is the process of demonstrating a chemical property, such as the burning match in Figure 1.2.2 "Chemical Properties". As the matter in the match burns, its chemical composition changes, and new forms of matter with new physical properties are created. Note that chemical changes are frequently accompanied by physical changes, as the new matter will likely have different physical properties from the original matter.
Example
Describe each process as a physical change or a chemical change.
Solution
Exercise
Identify each process as a physical change or a chemical change.
Answers
A sample of matter that has the same physical and chemical properties throughout is called a substance.Matter that has the same physical and chemical properties throughout.. Sometimes the phrase pure substance is used, but the word pure isn’t needed. The definition of the term substance is an example of how chemistry has a specific definition for a word that is used in everyday language with a different, vaguer definition. Here, we will use the term substance with its strict chemical definition.
Chemistry recognizes two different types of substances: elements and compounds.
A substance that cannot be broken down into simpler chemical substances by ordinary chemical means. is the simplest type of chemical substance; it cannot be broken down into simpler chemical substances by ordinary chemical means. There are about 115 elements known to science, of which 80 are stable. (The other elements are radioactive, a condition we will consider in Chapter 15.) Each element has its own unique set of physical and chemical properties. Examples of elements include iron, carbon, and gold.
A combination of more than one element. is a combination of more than one element. The physical and chemical properties of a compound are different from the physical and chemical properties of its constituent elements; that is, it behaves as a completely different substance. There are over 50 million compounds known, and more are being discovered daily. Examples of compounds include water, penicillin, and sodium chloride (the chemical name for common table salt).
Elements and compounds are not the only ways in which matter can be present. We frequently encounter objects that are physical combinations of more than one element or compound. Physical combinations of more than one substance are called mixtures. There are two types of mixtures.
A mixture composed of two or more substances. is a mixture composed of two or more substances. It is easy to tell, sometimes by the naked eye, that more than one substance is present.
A combination of two or more substances that is so intimately mixed that the mixture behaves as a single substance. is a combination of two or more substances that is so intimately mixed that the mixture behaves as a single substance. Another word for a homogeneous mixture is solution. Thus, a combination of salt and steel wool is a heterogeneous mixture because it is easy to see which particles of the matter are salt crystals and which are steel wool. On the other hand, if you take salt crystals and dissolve them in water, it is very difficult to tell that you have more than one substance present just by looking—even if you use a powerful microscope. The salt dissolved in water is a homogeneous mixture, or a solution (Figure

Figure
: Types of Mixtures © Thinkstock On the left, the combination of two substances is a heterogeneous mixture because the particles of the two components look different. On the right, the salt crystals have dissolved in the water so finely that you cannot tell that salt is present. The homogeneous mixture appears like a single substance.
Example
Identify the following combinations as heterogeneous mixtures or homogenous mixtures.
Solution
Exercise
Are the following combinations homogeneous mixtures or heterogeneous mixtures?
Answers
There are other descriptors that we can use to describe matter, especially elements. We can usually divide elements into metals and nonmetals, and each set shares certain (but not always all) properties.
An element that conducts electricity and heat well and is shiny, silvery, solid, ductile, and malleable. is an element that is solid at room temperature (although mercury is a well-known exception), is shiny and silvery, conducts electricity and heat well, can be pounded into thin sheets (a property called malleability), and can be drawn into thin wires (a property called ductility).
An element that exists in various colors and phases, is brittle, and does not conduct electricity or heat well. is an element that is brittle when solid, does not conduct electricity or heat very well, and cannot be made into thin sheets or wires (Figure 1.2.5 "Semimetals"). Nonmetals also exist in a variety of phases and colors at room temperature.
Some elements have properties of both metals and nonmetals and are called semimetals (or metalloids) - An element that has properties of both metals and nonmetals.. We will see later how these descriptions can be assigned rather easily to various elements.

Figure
: Semimetals © Thinkstock On the left is some elemental mercury, the only metal that exists as a liquid at room temperature. It has all the other expected properties of a metal. On the right, elemental sulfur is a yellow nonmetal that usually is found as a powder.
"Describing Matter" is a flowchart of the relationships among the different ways of describing matter.
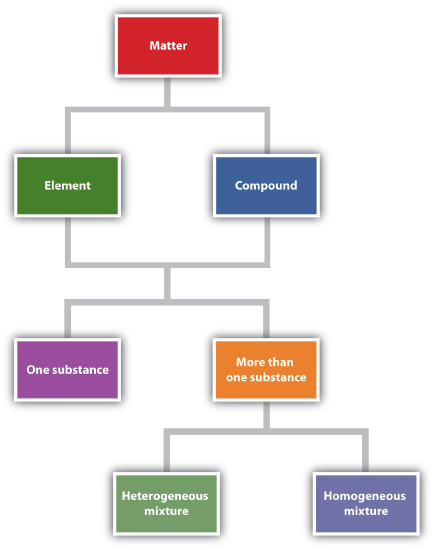
Figure
: Describing Matter. This flowchart shows how matter can be described.
Example
: Chemistry Is Everywhere: In the Morning
Most people have a morning ritual, a process that they go through every morning to get ready for the day. Chemistry appears in many of these activities.
These are just a few examples of how chemistry impacts your everyday life. And we haven’t even made it to lunch yet!
Figure
: Chemistry in Real Life © Thinkstock. Examples of chemistry can be found everywhere—such as in personal hygiene products, food, and motor vehicles.
Anonymous (by request)
Open Educational Resource created as part of the Digital Initiatives of the Brooklyn College Library